Popis produktu
SARS-CoV-2 Antigen Detection Kit (20 pcs)
QUICK TEST for effective screening in suspected cases of infection caused by the SARS-CoV-2 virus and thus COVID-19 infection with a result within 15 MINUTES. The Antigen Test is a rapid, colloidal gold-based immunochromatographic test for the qualitative determination of the Nucleocapsid Protein antigen (so-called N-protein) of SARS-CoV-2 virus from saliva or sputum, which serves as a rapid and effective diagnosis of COVID-19 infection.
Intended use:
For in vitro qualitative detection of SARSCoV-2 nucleocapsid antigen in nasal swab (NS) samples directly from suspected COVID-19 diagnosed by a healthcare provider within the first 5 days of symptom onset. This test is provided for use only by clinical laboratories or healthcare professionals for on-site testing, not for home testing purposes. Coronavirus 2 causing severe acute respiratory syndrome (SARSCoV-2 or 2019-nCoV) is an enveloped non-segmented RNA virus with positive polarity.
It causes coronavirus disease (COVID-19) transmissible among humans. SARS-CoV-2 has several structural proteins, including spike (S), envelope (E), membrane (M), and nucleocapsid (N). The antigen is generally detectable in samples from the upper respiratory tract in the acute phase of infection. Positive results indicate the presence of viral antigens, but a clinical correlation with the patient’s medical history and other diagnostic information is necessary to determine the infection status. Positive results do not rule out bacterial infection or co-infection caused by other viruses.
The identified agent may not be the clear cause of the disease. Negative results should be considered presumptive, ie, not excluding SARS-CoV-2 infection, and should not be used as the sole basis for patient treatment or treatment decisions, including infection control decisions. Negative results should be assessed in the context of recent patient exposure, history, and the presence of clinical signs and symptoms consistent with COVID-19, and should be confirmed by molecular analysis if necessary to treat the patient.
IVD Notification Certificate (Mod) Vazyme
Severe Acute Respiratory Syndrome Coronavirus
Test principle:
The rapid SARS-CoV-2 antigen test kit from Nanjing Vazyme Medical Technology Co., Ltd uses the IC method and was developed to detect the presence or absence of SARS-CoV-2 nucleocapsid proteins in respiratory tract samples from patients with signs and symptoms of suspected COVID- 19. Main components: anti-nucleocapsid protein and chicken IgY antibody with colloidal gold particles, nitrocellulose membrane coated with anti-nucleocapsid protein antibody and goat anti-chicken IgY antibody.
After processing the sample and introducing it into the test device, the SARS-CoV-2 antigens present in the sample bind to colloidal gold-conjugated antibodies on the test strip. The antigen conjugate complexes migrate along the test strip into the reaction region, where they are captured by a line of antibodies bound to the membrane. A colored line appears when the antigen conjugate is stored in test area „T“ and control area „C“ on the device.
Sampling and handling:
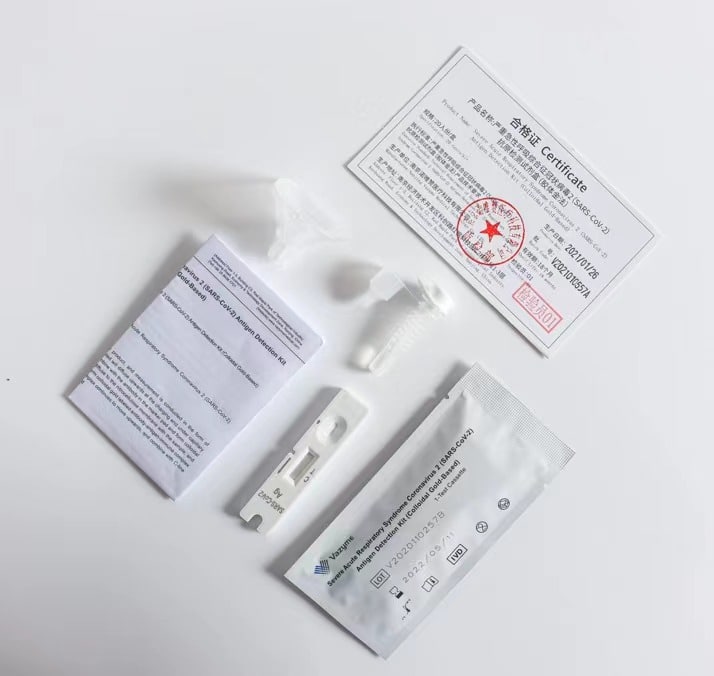
1. Specimen Collection and Preparation Acceptable specimens for testing with this kit include nasal swab specimens obtained by the two nostril sampling method. The correct sampling and preparation procedure must be followed. Samples obtained at an early stage after the onset of symptoms will contain the highest virus titer, samples taken after five days of symptoms will show a higher probability of negative results compared to the RTPCR analysis. Improper sample collection, improper sample handling, and / or transport may result in a false negative result. Therefore, training of sampling personnel is strongly recommended to ensure sample quality to achieve accurate testing results.
2. Transport and storage of samples Freshly collected samples should be processed as soon as possible, but no later than one hour after collection. Proper sampling and preparation methods must be followed.
3. Nasal swab sampling
4. What to DO and NOT to do when taking a sample:
– Take samples as soon as possible after the onset of symptoms.
– Test the samples immediately.
– Use only the swabs included in the kit.
– After sampling, do not return the swab to the swab package
Interpretation of results:
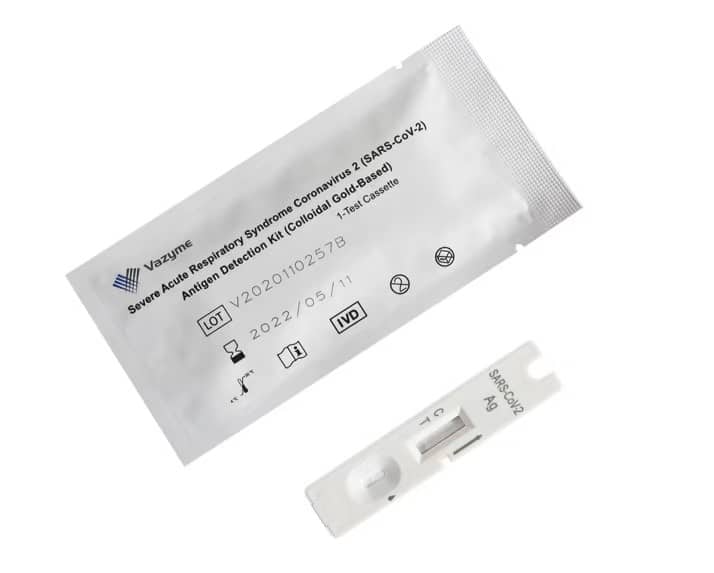
1. POSITIVE: Two lines will appear. One colored line should be in the area of the control line (C), the other colored line will appear in the area of the test line (T). A positive result indicates the presence of viral antigens, but a clinical correlation with the patient’s medical history and other diagnostic information is necessary to determine the status of the infection. Positive results do not rule out bacterial infection or co-infection with other viruses. The identified agent may not be the clear cause of the disease.
2. NEGATIVE: Only one colored line appears. Negative results are presumptive. A negative test result does not rule out infection and should not be used as the sole basis for determining treatment or other patient treatment decisions, including infection control decisions, especially in the presence of clinical signs and symptoms consistent with COVID-19 or in contacted patients. with a virus. It is recommended that these results be confirmed by a molecular test method if necessary to treat the patient.
3. INVALID: The control line does not appear. The most likely reasons why a control line does not appear are insufficient buffer or incorrect procedure techniques. Check the procedure and repeat with a new test cartridge. If the problem persists, stop using the test kit immediately and contact your local distributor.
4. Time of evaluation of the result: The result must be evaluated within 15 – 20 minutes from the addition of the sample to the sample space. The result displayed after 20 minutes is invalid.